Jul 18, 2023As a typical example of a buffer solution, let us consider the solution obtained when 3.00 mol acetic acid (HC 2 H 3 O 2) and 2.00 mol sodium acetate (Na C 2 H 3 O 2) are added to sufficient water to produce a solution of total volume 1 L.The stoichiometric concentration of acetic acid, namely, c a, is then 3.00 mol L -1, while the stoichiometric concentration of sodium acetate, c b, is 2.00
Buffers: Definition, Applications, & Industry Uses
Example \(\PageIndex1\) A 0.150 M solution of formic acid at 25°C (pKa = 3.75) has a pH of 2.28 and is 3.5% ionized. Is there a change to the pH of the solution if enough solid sodium formate is added to make the final formate concentration 0.100 M (assume that the formic acid concentration does not change)?

Source Image: phenomenex.blog
Download Image
Find step-by-step Chemistry solutions and your answer to the following textbook question: Indicate which of the following aqueous solutions are buffer solutions, and explain your reasoning. (b) 0.100 M NaCl – 0.100 M NH$_4$cL (c) 0.100 M CH$_3$NH$_2$ – 0.150 M CH$_3$NH$_3^+$Cl$^-$ (d) 0.100 M HCl – 0.050 M NaNO$_2$

Source Image: slideserve.com
Download Image
Using Alkaline Buffers for Calibrations is Not Without Risk – M4 Knick
Aqueous Equilibria: Part 1 – Buffers & Titrations … Of the following solutions, which has the greatest buffering capacity? A) 0.543 M NH 3 and 0.555 M NH 4 Cl B) 0.087 M NH 3 … Consider a buffer solution containing 0.100 M fluoride ions and 0.126 M hydrogen fluoride.

Source Image: yumpu.com
Download Image
Which Of The Following Aqueous Solutions Are Buffer Solutions
Aqueous Equilibria: Part 1 – Buffers & Titrations … Of the following solutions, which has the greatest buffering capacity? A) 0.543 M NH 3 and 0.555 M NH 4 Cl B) 0.087 M NH 3 … Consider a buffer solution containing 0.100 M fluoride ions and 0.126 M hydrogen fluoride.
Question Which of the following is a buffer solution? A CH3COOH+CH3COON a B N aCl+N aOH C HCl+N H4Cl D CH3COOH+HCl Solution Verified by Toppr A buffer solution is an aqueous solution of weak acid and its conjugate base.It can also be a mixture of weak base and its conjugate base.
Experiment 19 Acids, Bases, and Buffers rev 2/13
Question: Which of the following aqueous solutions are buffer solutions? (Select all that apply.) 0.22 M HI + 0.18 M Nal 0.29 M NH4 Br + 0.33 M NH3 D0.12 M HCN + 0.21 M KCN 0.32 M KNO3 + 0.27 M KBr 0.18 M Ca (OH)2 + 0.23 M CaBr2 Show transcribed image text Here’s the best way to solve it. Expert-verified
My LC Blog: LC Separation Modes

Source Image: linkedin.com
Download Image
Definitions of acids and bases: Video & Anatomy | Osmosis
Question: Which of the following aqueous solutions are buffer solutions? (Select all that apply.) 0.22 M HI + 0.18 M Nal 0.29 M NH4 Br + 0.33 M NH3 D0.12 M HCN + 0.21 M KCN 0.32 M KNO3 + 0.27 M KBr 0.18 M Ca (OH)2 + 0.23 M CaBr2 Show transcribed image text Here’s the best way to solve it. Expert-verified

Source Image: osmosis.org
Download Image
Buffers: Definition, Applications, & Industry Uses
Jul 18, 2023As a typical example of a buffer solution, let us consider the solution obtained when 3.00 mol acetic acid (HC 2 H 3 O 2) and 2.00 mol sodium acetate (Na C 2 H 3 O 2) are added to sufficient water to produce a solution of total volume 1 L.The stoichiometric concentration of acetic acid, namely, c a, is then 3.00 mol L -1, while the stoichiometric concentration of sodium acetate, c b, is 2.00

Source Image: excedr.com
Download Image
Using Alkaline Buffers for Calibrations is Not Without Risk – M4 Knick
Find step-by-step Chemistry solutions and your answer to the following textbook question: Indicate which of the following aqueous solutions are buffer solutions, and explain your reasoning. (b) 0.100 M NaCl – 0.100 M NH$_4$cL (c) 0.100 M CH$_3$NH$_2$ – 0.150 M CH$_3$NH$_3^+$Cl$^-$ (d) 0.100 M HCl – 0.050 M NaNO$_2$

Source Image: m4knick.com
Download Image
Solved Which of the following aqueous solutions are buffer | Chegg.com
Indicate which of the following aqueous solutions are buffer solutions, and explain your reasoning. [Hint: Consider any reactions that might occur between solution components.] (a) 0.100 \mathrm M \mathrm NaCl 0.100MNaCl Solution Verified Answered 1 year ago Create an account to view solutions By signing up, you accept Quizlet’s Privacy Policy

Source Image: chegg.com
Download Image
Which of the following acidic aqueous solutions are good buffer systems?
Aqueous Equilibria: Part 1 – Buffers & Titrations … Of the following solutions, which has the greatest buffering capacity? A) 0.543 M NH 3 and 0.555 M NH 4 Cl B) 0.087 M NH 3 … Consider a buffer solution containing 0.100 M fluoride ions and 0.126 M hydrogen fluoride.

Source Image: proprep.com
Download Image
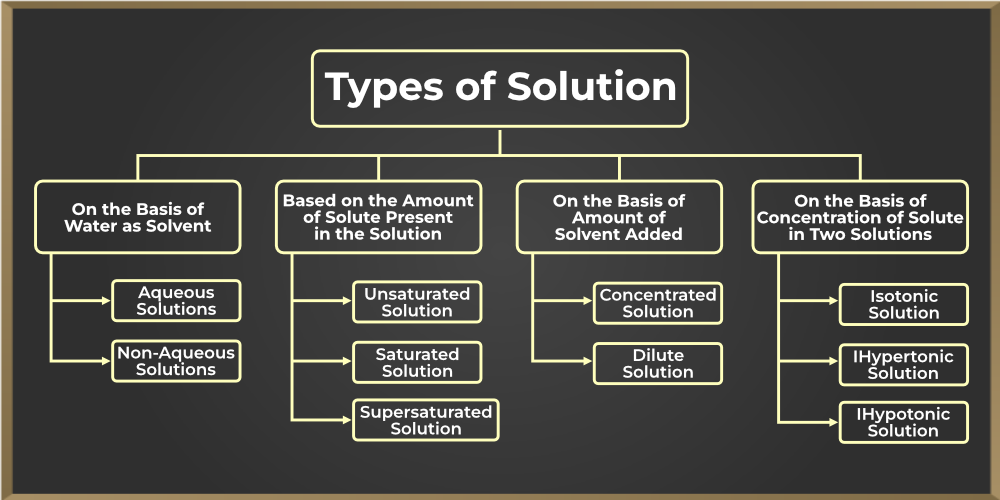
Solution – Definition, Types, Properties, Examples, and FAQs
Question Which of the following is a buffer solution? A CH3COOH+CH3COON a B N aCl+N aOH C HCl+N H4Cl D CH3COOH+HCl Solution Verified by Toppr A buffer solution is an aqueous solution of weak acid and its conjugate base.It can also be a mixture of weak base and its conjugate base.
Source Image: geeksforgeeks.org
Download Image
Definitions of acids and bases: Video & Anatomy | Osmosis
Solution – Definition, Types, Properties, Examples, and FAQs
Example \(\PageIndex1\) A 0.150 M solution of formic acid at 25°C (pKa = 3.75) has a pH of 2.28 and is 3.5% ionized. Is there a change to the pH of the solution if enough solid sodium formate is added to make the final formate concentration 0.100 M (assume that the formic acid concentration does not change)?
Using Alkaline Buffers for Calibrations is Not Without Risk – M4 Knick Which of the following acidic aqueous solutions are good buffer systems?
Indicate which of the following aqueous solutions are buffer solutions, and explain your reasoning. [Hint: Consider any reactions that might occur between solution components.] (a) 0.100 \mathrm M \mathrm NaCl 0.100MNaCl Solution Verified Answered 1 year ago Create an account to view solutions By signing up, you accept Quizlet’s Privacy Policy