Updated on June 02, 2019 The three parts of an atom are positive-charged protons, negative-charged electrons, and neutral neutrons. Follow these simple steps to find the number of protons, neutrons, and electrons for an atom of any element. Key Takeaways: Number of Protons, Neutrons, and Electrons Atoms are made of protons, neutrons, and electrons.
How to find the Number of Protons, Electrons, Neutrons for Iron (Fe) – YouTube
An ion of platinum has a mass number of 195 and contains 74 electrons. How many protons and neutrons does it contain, and what is its charge? Answers. 78 protons; 117 neutrons; charge is 4+ … Iron, 26 protons, 24 electrons, and 32 neutrons; (b) iodine, 53 protons, 54 electrons, and 74 neutrons . 4. 5. The atomic number is the number of

Source Image: studypool.com
Download Image
Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.

Source Image: topblogtenz.com
Download Image
Chemical Elements.com – Iron (Fe) Iron exists in its ionic form as either Fe²⁺ or Fe³⁺. These consist of a varying number of electrons but the same number of protons and neutrons. Fe²⁺ is formed from the loss of 2 valence electrons. Therefore, it has 26 – 2 = 24 electrons with 26 protons and 30 neutrons. Fe³⁺, on the other hand, is the ion resulting from the loss
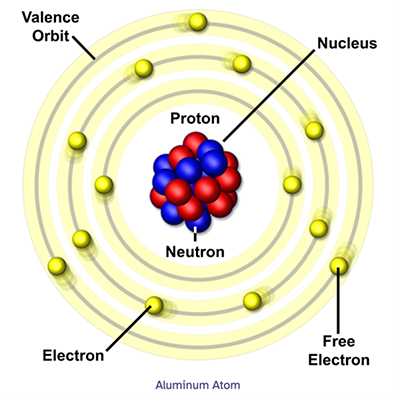
Source Image: nationalmaglab.org
Download Image
How Many Electrons Protons And Neutrons Does Iron Have
Iron exists in its ionic form as either Fe²⁺ or Fe³⁺. These consist of a varying number of electrons but the same number of protons and neutrons. Fe²⁺ is formed from the loss of 2 valence electrons. Therefore, it has 26 – 2 = 24 electrons with 26 protons and 30 neutrons. Fe³⁺, on the other hand, is the ion resulting from the loss Together, the number of protons and the number of neutrons determine an element’s mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number. A property closely related to an atom’s mass number is its atomic mass.
Atomic Ornament – Magnet Academy
Periodic Table of Videos Created by video journalist Brady Haran working with chemists at The University of Nottingham. Element Iron (Fe), Group 8, Atomic Number 26, d-block, Mass 55.845. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. 3.atomic Structure | PDF

Source Image: scribd.com
Download Image
Iron Bohr Model – How to draw Bohr diagram for Iron(Fe) Periodic Table of Videos Created by video journalist Brady Haran working with chemists at The University of Nottingham. Element Iron (Fe), Group 8, Atomic Number 26, d-block, Mass 55.845. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.

Source Image: topblogtenz.com
Download Image
How to find the Number of Protons, Electrons, Neutrons for Iron (Fe) – YouTube Updated on June 02, 2019 The three parts of an atom are positive-charged protons, negative-charged electrons, and neutral neutrons. Follow these simple steps to find the number of protons, neutrons, and electrons for an atom of any element. Key Takeaways: Number of Protons, Neutrons, and Electrons Atoms are made of protons, neutrons, and electrons.

Source Image: m.youtube.com
Download Image
Chemical Elements.com – Iron (Fe) Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
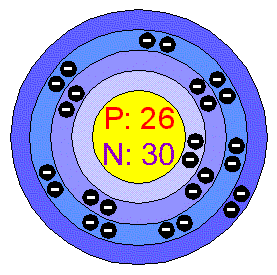
Source Image: chemicalelements.com
Download Image
What Are an Atom, Electron, Neutron and Proton? | Sciencing Jul 29, 2022Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74). Since the iodine is added as a 1− anion, the number of electrons is 54 [53 – (1-) = 54]. Exercise 2.6.1 2.6. 1. An ion of platinum has a mass number of 195 and contains 74 electrons.

Source Image: sciencing.com
Download Image
How to find Protons & Electrons for Fe2+ and Fe3+ (Iron II and III ions) – YouTube Iron exists in its ionic form as either Fe²⁺ or Fe³⁺. These consist of a varying number of electrons but the same number of protons and neutrons. Fe²⁺ is formed from the loss of 2 valence electrons. Therefore, it has 26 – 2 = 24 electrons with 26 protons and 30 neutrons. Fe³⁺, on the other hand, is the ion resulting from the loss

Source Image: m.youtube.com
Download Image
What is the charge on the electron, the proton, and the neutron, respectively? , +1, +1 -1, +1, -1 -1, 0, +1 -1, +1, 0 +1, +1, ppt video online download Together, the number of protons and the number of neutrons determine an element’s mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number. A property closely related to an atom’s mass number is its atomic mass.

Source Image: slideplayer.com
Download Image
Iron Bohr Model – How to draw Bohr diagram for Iron(Fe)
What is the charge on the electron, the proton, and the neutron, respectively? , +1, +1 -1, +1, -1 -1, 0, +1 -1, +1, 0 +1, +1, ppt video online download An ion of platinum has a mass number of 195 and contains 74 electrons. How many protons and neutrons does it contain, and what is its charge? Answers. 78 protons; 117 neutrons; charge is 4+ … Iron, 26 protons, 24 electrons, and 32 neutrons; (b) iodine, 53 protons, 54 electrons, and 74 neutrons . 4. 5. The atomic number is the number of
Chemical Elements.com – Iron (Fe) How to find Protons & Electrons for Fe2+ and Fe3+ (Iron II and III ions) – YouTube Jul 29, 2022Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74). Since the iodine is added as a 1− anion, the number of electrons is 54 [53 – (1-) = 54]. Exercise 2.6.1 2.6. 1. An ion of platinum has a mass number of 195 and contains 74 electrons.