Name: Oxirane IUPAC Systematic Name: Oxirane Synonyms: Dihydrooxirene; dimethylene oxide; 1,2-epoxyethane; epoxyethane; ethene oxide; EtO; ETO; oxacyclopropane; oxane; oxidoethane 1.1.2. Structural and molecular formulae and relative molecular mass 1.1.3. Chemical and physical properties of the pure substance
The mechanism of the catalytic oxidation of ethylene – II. Reactions between ethylene, etc. and chemisorbed oxygen monolayers
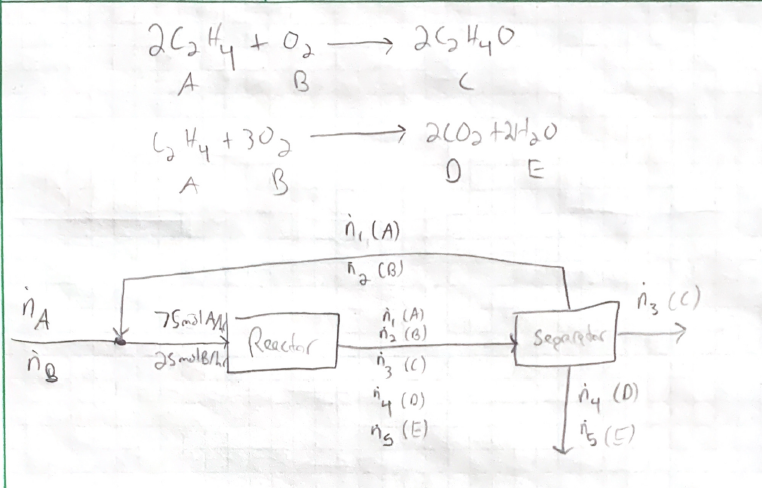
Ethylene oxide is produced by the catalytic oxidation of ethylene: 2C2H4 + O2 2C2H4O An undesired competing reaction is the combustion of ethylene C2H4 + 302-2C02 + 2H20 The feed to the reactor (not the fresh feed to the process) contains 3 moles of ethylene per mole of oxygen-The single-pass conversion of ethylene is 20%, and for every 100 mole
Source Image: medium.com
Download Image
May 1, 2022Commercially ethylene oxide is produced by direct catalytic ethylene oxidation with air or oxygen supported over to catalyst silver and additional selectivity. … Ethylene is oxidized in the catalytic environment, and ethylene oxide produces other water and carbon dioxide CO 2 products. This oxidation reaction is very disruptive, so cooling

Source Image: toppr.com
Download Image
Solved) – Figure SAT6.4P2 shows a simplified process to make ethylene… (1 Answer) | Transtutors Ethylene oxide is produced by the catalytic oxidation of ethylene over a silver-containing catalyst. A side reaction oxidizes ethylene to carbon dioxide and water. CH + 0 . 5 O → C H 2 4 O ζ1 2 4 2 CH + 3 O → 2 4 2 2 CO + 2 2 H O 2 ζ2 where ζi is the extent of reaction of reaction i.

Source Image: pubs.acs.org
Download Image
Ethylene Oxide Is Produced By The Catalytic Oxidation Of Ethylene
Ethylene oxide is produced by the catalytic oxidation of ethylene over a silver-containing catalyst. A side reaction oxidizes ethylene to carbon dioxide and water. CH + 0 . 5 O → C H 2 4 O ζ1 2 4 2 CH + 3 O → 2 4 2 2 CO + 2 2 H O 2 ζ2 where ζi is the extent of reaction of reaction i. Ethylene oxidation by Ag catalysts has been extensively investigated over the past few decades, but many key fundamental issues about this important catalytic system are still unresolved.
Continuous Liquid-Phase Epoxidation of Ethylene with Hydrogen Peroxide on a Titanium-Silicate Catalyst | Industrial & Engineering Chemistry Research
Engineering Chemical Engineering Chemical Engineering questions and answers 4. Ethylene oxide is produced by the catalytic oxidation of ethylene. 2C2H4+O2→2C2H4O An undesired reaction is the combustion of ethylene C2H4+3O2→2CO2+2H2O The feed to the reactor contains 3 moles of ethylene per mole of oxygen. Ethylene Oxide Production by Seval Ak

Source Image: prezi.com
Download Image
WO2018029189A1 – Catalyst for the oxidation of ethylene to ethylene oxide – Google Patents Engineering Chemical Engineering Chemical Engineering questions and answers 4. Ethylene oxide is produced by the catalytic oxidation of ethylene. 2C2H4+O2→2C2H4O An undesired reaction is the combustion of ethylene C2H4+3O2→2CO2+2H2O The feed to the reactor contains 3 moles of ethylene per mole of oxygen.

Source Image: patents.google.com
Download Image
The mechanism of the catalytic oxidation of ethylene – II. Reactions between ethylene, etc. and chemisorbed oxygen monolayers Name: Oxirane IUPAC Systematic Name: Oxirane Synonyms: Dihydrooxirene; dimethylene oxide; 1,2-epoxyethane; epoxyethane; ethene oxide; EtO; ETO; oxacyclopropane; oxane; oxidoethane 1.1.2. Structural and molecular formulae and relative molecular mass 1.1.3. Chemical and physical properties of the pure substance
Source Image: royalsocietypublishing.org
Download Image
Solved) – Figure SAT6.4P2 shows a simplified process to make ethylene… (1 Answer) | Transtutors May 1, 2022Commercially ethylene oxide is produced by direct catalytic ethylene oxidation with air or oxygen supported over to catalyst silver and additional selectivity. … Ethylene is oxidized in the catalytic environment, and ethylene oxide produces other water and carbon dioxide CO 2 products. This oxidation reaction is very disruptive, so cooling
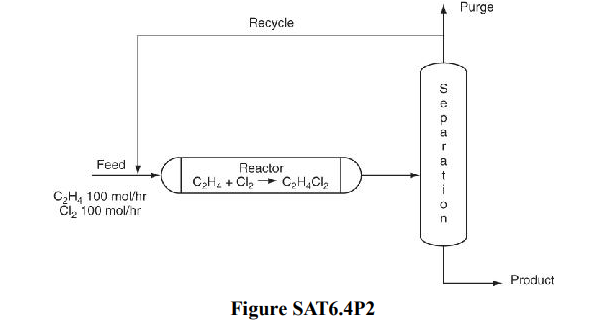
Source Image: transtutors.com
Download Image
Automated synthesis of oxygen-producing catalysts from Martian meteorites by a robotic AI chemist | Nature Synthesis The major application of ethylene oxide is in the manufacture of ethylene glycol, which accounts for more than 70% of the total ethylene oxide consumption (in 2009). The production of ethoxylates consumes another 11%, and smaller amounts are used to make higher glycols, ethanolamines, glycol ether, and polyols [1 ].
Source Image: nature.com
Download Image
Solved] Ethylene oxide is produced by the catalytic oxidation of ethylene:… | Course Hero Ethylene oxide is produced by the catalytic oxidation of ethylene over a silver-containing catalyst. A side reaction oxidizes ethylene to carbon dioxide and water. CH + 0 . 5 O → C H 2 4 O ζ1 2 4 2 CH + 3 O → 2 4 2 2 CO + 2 2 H O 2 ζ2 where ζi is the extent of reaction of reaction i.
Source Image: coursehero.com
Download Image
Ethylene oxide is produced by the catalytic oxidation of eth | Quizlet Ethylene oxidation by Ag catalysts has been extensively investigated over the past few decades, but many key fundamental issues about this important catalytic system are still unresolved.
Source Image: quizlet.com
Download Image
WO2018029189A1 – Catalyst for the oxidation of ethylene to ethylene oxide – Google Patents
Ethylene oxide is produced by the catalytic oxidation of eth | Quizlet Ethylene oxide is produced by the catalytic oxidation of ethylene: 2C2H4 + O2 2C2H4O An undesired competing reaction is the combustion of ethylene C2H4 + 302-2C02 + 2H20 The feed to the reactor (not the fresh feed to the process) contains 3 moles of ethylene per mole of oxygen-The single-pass conversion of ethylene is 20%, and for every 100 mole
Solved) – Figure SAT6.4P2 shows a simplified process to make ethylene… (1 Answer) | Transtutors Solved] Ethylene oxide is produced by the catalytic oxidation of ethylene:… | Course Hero The major application of ethylene oxide is in the manufacture of ethylene glycol, which accounts for more than 70% of the total ethylene oxide consumption (in 2009). The production of ethoxylates consumes another 11%, and smaller amounts are used to make higher glycols, ethanolamines, glycol ether, and polyols [1 ].